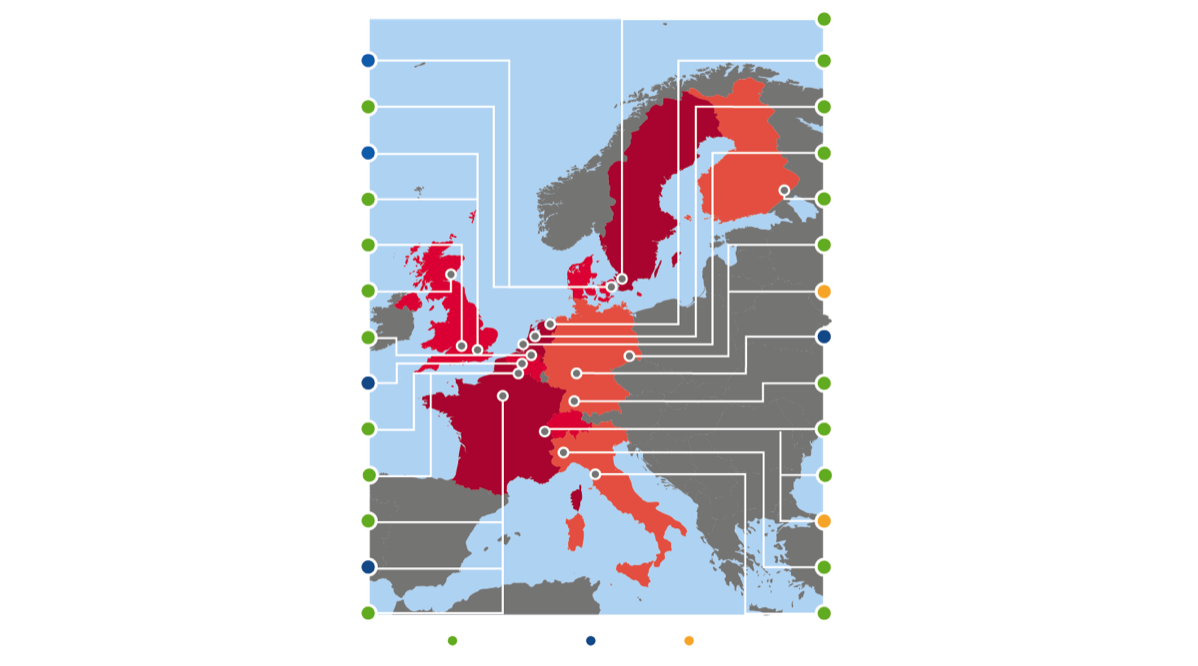
The RHAPSODY Consortium
Leading European experts from 20 academic institutions, 5 EFPIA (European Federation of Pharmaceutical Industries and Associations) pharmaceutical organisations and two SMEs have collaborated closely in the Innovative Medicines Initiative (IMI) project RHAPSODY. IMI is a unique Public Private Partnership between the pharmaceutical industry (represented by the EFPIA) and the European Union. The RHAPSODY project has received funding of more than EUR 18 million through the IMI2 programme, which includes an EC contribution of around EUR 8 million, matching the dedicated resources provided by participating pharma companies, as well as funding from the Swiss State Secretariat for Education, Research and Innovation (SERI).
Academia
University of Lausanne (UNIL) was created in 1537 and currently includes 8 faculties, with the Faculty of Biology and Medicine being the largest. It comprises a University Hospital with a very large patient base and active clinical and basic research, and a large Fundamental Sciences section active in teaching and research, with particular emphasis on neurosciences, immunology and cancer, pharmacology, and metabolism and metabolic diseases. The research activities are supported by numerous technical platforms and bioinformatics supports through the integration within the UNIL of the Swiss Institute of Bioinformatics. UNIL has extensive interactions with other Swiss, European and world-wide teaching and research institutions through a variety of national and international programs.
UNIL is the Coordinator of RHAPSODY. It contributes through its expertise in beta-cell biology and integrated physiological analysis of preclinical models to the elucidation of the role of specific genes and gene modules in beta-cells and insulin target tissue function. It is also involved in the generation of a whole body integrated transcriptomic model of pre-diabetes development.
The Lund University Diabetes Centre (LUDC) is a Swedish Centre of Excellence founded in 2006 and one of the leading diabetes centres in the world. They are located at the Clinical Research Centre of the University, Malmö University Hospital, which is closely connected to the Clinic of Diabetes and Endocrinology, creating the ideal setting for both basic and clinical research. The centre combines the university’s expertise in endocrinology, diabetes genetics, pancreatic β-cells, inflammation and (cardio)vascular diabetes complications.
Two research groups of ULUND contribute to RHAPSODY.
ULUNDa is academic co-coordinator of WP1 (Management, communications, sustainability and ethics) and contributes with data from extensively characterised clinical cohorts from Sweden and Finland, and also from human islets analyses and from the IMI SUMMIT-project.
ULUNDb is academic co-lead for WP3 (Predictive biomarkers of glycaemic deterioration) and lead of WP7 (Regulatory consensus). ULUNDb contributes to WP3, and to WP2 (for data harmonization) and WP5 (for causal inference and prediction modelling) with data analysis expertise and with large, well-characterized prospective cohorts from Sweden.
ULUNDa is academic co-coordinator of WP1 (Management, communications, sustainability and ethics) and contributes with data from extensively characterised clinical cohorts from Sweden and Finland, and also from human islets analyses and from the IMI SUMMIT-project.
ULUNDb is academic co-lead for WP3 (Predictive biomarkers of glycaemic deterioration) and lead of WP7 (Regulatory consensus). ULUNDb contributes to WP3, and to WP2 (for data harmonization) and WP5 (for causal inference and prediction modelling) with data analysis expertise and with large, well-characterized prospective cohorts from Sweden.

- Lund, Sweden
- Prof. Dr. Leif Groop (ULUNDa)
- Prof. Dr. Paul Franks (ULUNDb)
The Paul Langerhans Institute Dresden (PLID) of Helmholtz Zentrum München is part of the Technische Universität Dresden (TUD), one of eleven German “Universities of Excellence” and partner in three German Centers for Health Research (DZG). With ≈37,000 students, >9,000 employees and 520 professors it is the largest university in Saxony encompassing 14 departments. TUD is the No1 university for issuing patents in Germany, and Dresden is only second to Munich in the density of scientific institutions, hosting 3 Max Planck, 3 Leibniz and 11 Fraunhofer, Institutes and also a Helmholtz Center.
TUD leads WP5 (Predictive biomarkers of beta cell dysfunction), collecting pancreatic tissue/islets from non-diabetic, pre-diabetic and T2D partially pancreatectomized patients. It also conducts -omics and imaging studies to discover mechanisms leading to impaired insulin secretion and beta cell dysfunction and biomarkers of T2D disease progression.
The Università di Pisa (UNIPI), is a public institution and one of the oldest Universities in the world (founded in 1343). It counts several Nobel Prize winners among its alumni. UNIPI has more than 52,000 students, almost 800 doctoral students and 1,100 students enrolled in its approximately 50 residency programmes in the medical and health areas or in specialisation courses in other disciplines. The University interacts closely with the local public Santa Chiara – Cisanello Hospital, providing research and clinical training.
UNIPI contributes by:
- sharing previous data and biorepository samples of pancreatic tissue and isolated islets from non-diabetic and type 2 diabetic donors;
- preparing and characterizing isolated human islets, to be studied functionally and molecularly under different conditions, in order to discover and validate biomarkers of beta cell failure
- studying human liver samples and peripancreatic adipose tissue specimens, to identify insulin target tissue biomarkers
Situated in the heart of Paris, Université de Paris (UP) is a major actor in European higher education and research. The UP is a research partner for both the CNRS (French National Centre for Scientific Research) and INSERM (French National Institute for Health and Medical Research); and its research is applied through contracts, patents and business incubators. Two medical schools (Bichat and Lariboisière) with excellent diabetes departments are affiliated with the UP, allowing research training for medical students and collaborations in clinical/basic diabetes research.
UP is WP6 (Predictive biomarkers of insulin target tissue dysfunction) co-leader and has two main tasks:
- circulating biomarkers, which reflect development of insulin resistance and changes in insulin target tissue transcripts and proteins at defined steps in the progression to pre-diabetes and T2D;
- genes, gene expression modules, and epigenetic marks deregulated in insulin target tissues with causal links to impaired beta-cell function, which could represent new targets for drug or lifestyle interventions.
INSERM is the French National Institute of Health and Medical Research. Its mission is to facilitate exchanges between basic and clinical research, to improve the understanding of human diseases and to ensure that patients benefit rapidly from the latest research findings. INSERM has a strong track record in the field of diabetes and particularly of type 2 diabetes. Inserm is also affiliated with the Cochin Institue, a research center (no need for the capital letters for those two words) also affiliated with CNRS and Paris Descartes University. It is composed of: ~620 staff members, including scientists, University teachers and clinicians; 36 research groups; and 10 state-of-the-art core facilities. Covering a surface of ~14,000 m2, it is located on the Cochin Hospital campus, in close proximity to the largest Clinical Department of Adult Diabetology in greater Paris.
INSERM studies and models functional beta cell mass deficiency that takes place in patients with Type 2 diabetes. Innovative models and read-outs will be developed to reach the objective to be able to understand, treat and prevent diabetes.
The Université Libre de Bruxelles (ULB), with its five Nobel Prizes, three Wolf Prizes for physics, a Fields medal and two Abel prizes in mathematics, is an internationally recognized research center. The ULB Center for Diabetes Research (UCDR) - partner ULB in the project - interacts closely with the ULB Erasmus Hospital, providing research training for students/residents and collaborating in clinical/basic diabetes research.
ULB performs RNA-seq transcriptome profiling and functional genomics studies of metabolically stressed human islets and islets from type 2 diabetic organ donors, with the goal to understand β cell dysfunction and death in T2D and to discover novel biomarkers for these processes, including circulating human islet- or β cell-specific epigenetic marks and splice variants.
The Swiss Institute of Bioinformatics (SIB) is an academic, non-profit foundation of public utility established in 1998. SIB coordinates research and education in bioinformatics throughout Switzerland and provides high quality bioinformatics services to the international research community. SIB is well known for its life science resources, such as UniProtKB/Swiss-Prot, SWISS-MODEL, STRING, ExPASy etc. and comprises more than 56 research and service groups in Swiss universities, technical (ETH/EPF) and applied universities (HES). SIB also has a long history in developing high-quality and high-performance software (the latter particularity via the Vital-IT competency centre).
SIB leads WP2 (Data federation and systems biology) to set up a federated system for IMI1/IMI2 data mining via a central web portal. It also enacts integrative systems-level analysis of genomics, metabolomics and clinical data for biomarker prioritization and annotate and format data and results according to clinical (CDISC) and other relevant standards.
The University of Oxford (UOXF) is a leading academic institution, at the forefront of scientific research, and consistently rated amongst the world’s best universities. Oxford was recently ranked top in the UK for overall quality of submissions in Clinical Medicine by the UK research assessment exercise. Within Biological Sciences, Oxford produced the largest volume of world leading and internationally excellent research. Oxford is also one of the world’s leading centres for the application of advanced statistical and computational approaches to human genetic and genomic data. RHAPSODY project partners from the University of Oxford are based in the Oxford Centre for Diabetes, Endocrinology and Metabolism and the Wellcome Trust Centre for Human Genetics.
UOXFa co-leads WP5 (Predictive biomarkers of beta cell dysfunction), providing access to both islet samples and genomic data, to large human genetics resources (GWAS, sequencing), and performing integrative analyses. UOXFa is also providing genomics expertise, analytics to WP2 (Data federation and systems biology), and access to cohorts and data. Furthermore, UOXFa is the partner of contact with OR and facilitates communication with DIRECT, SUMMIT, STEMBANCC and Accelerating Medicines Partnership in the US that will support implementation of RHAPSODY goals.
UOXFb leads WP8 (Modelling economic and public health impact of disease modification) and develops, integrates and validates economic models of pre-diabetes and type 2 diabetes, assess early health technology of selected case studies, and will engage with other researchers, patient groups and policy makers on health economics. IUOXFb also contributes to the data harmonization required to estimate the economic models.
UOXFb leads WP8 (Modelling economic and public health impact of disease modification) and develops, integrates and validates economic models of pre-diabetes and type 2 diabetes, assess early health technology of selected case studies, and will engage with other researchers, patient groups and policy makers on health economics. IUOXFb also contributes to the data harmonization required to estimate the economic models.
Centre National de la Recherche Scientifique (CNRS) is the major French research multidisciplinary institution. The Lille CNRS UMR 8189 unit specializes in “integrated genomics and modeling of metabolic diseases” and is located in the Lille Pasteur Institute campus as part of the laboratory of excellence, “Lille European Genomic Institute for Diabetes” (EGID, http://www.egid.fr/), which is responsible for the genomic equipment of excellence and LIGAN-personalized medicine.
CNRS contributes primarily through genomic, epigenomic, and expression analyses in WP4 (Nanostring mRNA/microRNA analysis in plasma), WP5 (RNASeq, Methylation analyses and bio statistics, human beta cell line studies), and WP6 (RNASeq, Methylation analysis in human insulin sensitive tissues and statistics).
The participants from the University of Eastern Finland (UEF) are members of the Centre of Excellence of the Academy of Finland (‘Centre of Excellence in Cardiovascular and Metabolic Diseases’, funding for the years from 2014 to 2019) working at the Institute of Clinical Medicine at the UEF and the Kuopio University Hospital. The centre brings together the university’s expertise on type 2 diabetes (genetics, pathophysiology, metabolism, biomarkers, population-based studies, and cardiovascular complications). Research facilities include the Genome Centre of Eastern Finland for genetic studies, Clinical Research Centre of the UEF for population and metabolic studies, supported by a chemistry laboratory. Molecular biology laboratory for diabetes studies is located in the Canthia Building of the UEF.
UEF contributes with data from the deeply phenotyped METSIM cohort including 10,197 Finnish men, and identification and validation of biomarkers of glycemic deterioration before the onset of diabetes. UEF will furthermore contribute to discovery of candidate biomarkers for diabetes progression.
The University of Dundee (UNIVDUN) is internationally recognised for its expertise across a range of disciplines including science, medicine, engineering and art and is home to 18,000 students and more than 3000 staff. It has a reputation for excellence in research and continues to successfully attract leading researchers from across the world. The University is a recognised world leader in the fields of cancer, diabetes, drug metabolism and toxicology.
UNIVDUN is co-lead for WP3 (Predictive biomarkers of glycaemic deterioration), which involves coordinating access to cohort data and samples, developing models of progression, and analysing biomarkers in relation to these models. UNIVDUN provides access (via a federated database model) to a diabetes cohort called GoDARTS (Colin Palmer PI), and will work on statistical and genomic analysis of the progression data (Kaixin Zhou).
Imperial College London (Imperial) is a science-based research-intensive university recently ranked in the top two universities in the world (QS world rankings). The Section of Cell Biology and Functional Genomics is located in the state-of-the-art Imperial Centre for Translational and Experimental Medicine. The College boasts a broad spectrum of diabetes expertise ranging from human population genetics to basic cell biology and technology development.
Imperial co-leads WP4 (Multi-omics biomarker discovery and assay development), coordinating omics analysis of serum and plasma samples, and develop new imaging and electrophysiology approaches in cell lines and primary tissues to assess biological effects of selected biomarkers. Imperial also participates in development of new diagnostic tools. Imperial also contributes to bioinformatics analysis of large data sets.
Eberhard Karls Universität Tübingen (EKUT) is a medical school and university hospital, and is particularly focussed upon treatment of patients with diabetes and performing clinical and cohort studies.
EKUT contributes via collection and integration of data from human islets and related blood samples for the identification of candidate biomarkers of beta cell dysfunction and death, in relationship to potential pathogenic mechanisms. EKUT is particularly interested in validating the link between metabolic pathways in insulin-sensitive tissues and the production of plasma biomarkers.
- Tübingen, Germany
- Prof. Dr. Hans-Ulrich Häring
- Prof. Dr. Susanne Ullrich
Lipotype is a spin-off company from laboratories of Kai Simons and Andrej Shevchenko in the world-renowned Max-Planck-Institute of Molecular Cell Biology and Genetics in Dresden, Germany. Drawing on many years of cutting-edge research experience, Lipotype delivers comprehensive, absolutely quantitative lipid analysis services for clinical and biological samples on a high-throughput scale. Lipotype offers high quality lipid analysis services for a wide range of customers and applications, including biomarker identification for clinical researchers, pharma and biotech companies, functional food development for the food industry, and also for the small-scale profiling needs of academic researchers.
Lipotype will establish the lipid profile of blood plasma or serum samples from up to 3,000 pre-diabetic subjects in well-characterised cohorts, as a predictor of progression to T2D, by Lipotype Shotgun Lipidomics Technology. Samples will be assessed for over 2000 lipids, including 3 candidate lipids selected as predictive for T2D.
Kobenhavns Universitet (UCPH) carries out multi-disciplinary research within bioinformatics and systems biology with a particular focus on large data sets of relevance to human health, and includes the NNF Center for Protein Research and the Research Programme for Disease Systems Biology at the University of Copenhagen Medical School. World-wide UCPH is currently #35, 45, and 45 on the Shanghai, QS World and Leiden rankings, respectively.
UCPH is involved in general data management in establishing and maintaining the federated data infrastructure, interface to the DIRECT data warehouse, and data integration across partners. UCPH also provides advice on analysis of data generated within the consortium, also in conjunction with those available in public databases. Additionally, systems biology level analyses, methods development, in particular cell/tissue specific analysis of T2D relevant data, network rewiring analysis of disease progression patterns, and finally creation of re-ranked lists of targets and relevant subnetworks of genes and proteins based on input and ranking efforts.
Azienda Ospedaliero-Universitaria Città della Salute e dell Scienza di Torino (AOU TO) is part of the main Italian epidemiological centres, in terms of number of publications, impact factor and amount of funding received for research, and it is involved in a broad spectrum of activity within epidemiological research: clinical, environmental, etiological, occupational epidemiology, clinical trials, healthcare prevention, economic evaluation and development of methods. The Unit belongs to the Department of Medical Sciences of the University of Turin and has a long-lasting scientific collaboration with the Medical Units and the Piedmont Diabetes Register.
AOU TO models economic and public health impact of disease.
The Academisch Ziekenhuis Groningen (UMCG) is one of the largest hospitals in the Netherlands. More than 10,000 employees provide patient care, are involved in medical education and perform cutting-edge scientific research, focused on ‘healthy and active ageing.’ Research at the UMCG is characterized by a combination of fundamental and patient-oriented clinical research. The interaction between these two stimulates the development of new clinical and research opportunities. The Health Technology Assessment (HTA) unit of the Epidemiology department has Health Economic (HE) decision modelling as one of its main topics, and UMCG is thus an expert in such patient-level modelling.
UMCG contributes by first integrating the pre-diabetes and diabetes HE models and validate them, and then use the models to evaluate the potential cost-effectiveness of selected case studies.
The University Hospital of Lille (CHRU-Lille) is a bench-mark university hospital for primary care, teaching, innovation and research, serving the four million inhabitants of the Nord-Pas-de-Calais region. CHRU-Lille was study sponsor in 2013 of 141 clinical studies and participated in 147 clinical studies (institutional sponsorship) and 220 studies (industrial sponsorship). It is third in the national ranking (2013 SIGREC figures), and fourth in the national ranking regarding scientific publications (2010-2013).
CHRU Lille conducts a clinical trial in diabetic patients undergoing metabolic surgery, and collecting biological samples for « omics » studies to predict and explain deterioration of glucose control in T2D patients who had early remission after gastric bypass surgery.
Leiden University Medical Center (LUMC) has an outstanding scientific environment, providing state-of-art research facilities and a highly integrative network of life science groups seeking to improve the quality of healthcare. Diabetes research at the LUMC is strongly embedded within several research profiles at the LUMC including the profiles Ageing and Vascular and Regenerative Medicine. Furthermore, the LUMC has a longstanding intensive collaboration with several other RHAPSODY partners and especially, the VU University Medical Center (VUmc) Amsterdam, regarding collaborative research in type 2 diabetes.
LUMC will provide, together with VUmc, access to a population-based cohort with longitudinal data on progression towards diabetes (The new Hoorn study, n=2700) and a large cohort of type 2 diabetic subjects with longitudinal data on disease progression and development of diabetic complications (the Diabetes Care System West-Friesland (DCS), n=6000). Furthermore, LUMC will participate in projects using the different datasets in systems biology approaches towards precision medicine.
Founded in 2005, SCIPROM is an SME specialised in the management of collaborative research projects. SCIPROM supports coordinators from the first project idea to the final report, in project set-up, negotiation, management and communication. At present, SCIPROM has 7 employees.
SCIPROM is part of the Executive Committee and contributes to Project Management, Communication and Dissemination:
- Project office, contact point and support for the coordinator and all partners of the consortium
- Contractual, financial and legal management (Consortium Agreement, EU contract negotiations and amendments, IPR issues, supervision of financial reporting and auditing)
- Scientific editing, compilation of scientific and financial reports, collection of audit certificates
- Co-organisation of project meetings and workshops/satellite events
- Communication: Creation and maintenance of the project web site; project brochure
Servier is an independent French research-based pharmaceutical company with an international presence in 146 countries. Its development is driven by the pursuit of innovation in the therapeutic areas of cardiovascular, metabolic, central nervous system, psychiatric, bone, muscle and joint diseases, and also cancer. Servier engagement in research on diabetes is stressed by the strong internal research on insulin secretion and action and its expertise in development of anti-diabetic drugs. Moreover, 28% of turnover from Servier drugs was reinvested in Research and Development in 2014. Servier has a long history of collaboration with international institutions, particularly in research projects focused on diabetes.
Servier is Project Leader, Co-leader of WP1, and member of the Executive Committee. Servier is providing financial support and the in vivo and in vitro study of insulin action, measurement of plasma metabolites, substrates and hormones. Servier is also characterizing of animal models and financial support to “omic” studies).
Janssen Pharmaceuticals, Inc., a pharmaceutical company of Johnson & Johnson, provides medicines for an array of health concerns in several therapeutic areas, including diabetes. As part of Janssen’s internal efforts against diabetes, there is an emphasis on obtaining a better understanding of the patient population through the use of biomarkers.
Janssen provides scientific input and financial support for the analysis of biomarkers.

- Beerse, Belgium
- Dr. Patricia Andrade-Gordon
- Dr. Rachel Adams
- Dr. Scott Rairigh
- Dr. Cheryl Neslusan
Headquartered in Denmark, Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. The company also has leading positions within haemophilia care, growth hormone therapy and hormone replacement therapy. Novo Nordisk employs approximately 41,500 employees in 75 countries, and markets its products in more than 180 countries.
Novo Nordisk contributes to the development and maintenance of the RHAPSODY federated database and models trajectories of risk factors prior to diabetes. Novo Nordisk also contributes to the metabolomics analysis of samples and is involved in the validation of biomarkers and regulatory considerations for diabetes disease modification.
Sanofi is a leading global pharmaceutical company that discovers, develops and distributes therapeutic solutions to improve the lives of everyone (sanofi.com). Sanofi is one of the key drug suppliers for the treatment of diabetes patients worldwide. The strong engagement of Sanofi in diabetes research and development is reflected by coordinating and leading several IMI diabetes projects (IMIDIA, DIRECT and INNODIA) on pancreatic beta-cell research, development and progression of diabetes in subjects with pre-diabetes and patients with early onset diabetes, and the interplay between auto-immune response and pancreatic beta-cells in the development of type 1 diabetes.
Sanofi is co-leader for both WP3 and WP5. Sanofi’s input and contribution include beta cell characterisation, transcription profiling and biomarker discovery.
The VU University Medical Center's (VUmc's) core objectives are to ensure patient care in close collaboration with scientific research, academic teaching & post-academic training. Research within five focus areas is organized in five research institutes. Diabetes research is mainly embedded within the EMGO+ Institute for Public Health, which is renowned amongst others for a number of large cohort studies.
VUmc provides, together with partner 20, LUMC, access to both a population-based cohort with longitudinal data on progression towards diabetes (The new Hoorn study, n=2700) and a large cohort of type 2 diabetic subjects with longitudinal data on disease progression and development of diabetic complications (the Diabetes Care System West-Friesland (DCS), n=8500).
Lilly is a global healthcare leader that unites caring with discovery to make life better for people around the world. We were founded more than a century ago by a man committed to creating high-quality medicines that meet real needs, and today we remain true to that mission in all our work. Across the globe, Lilly employees work to discover and bring life-changing medicines to those who need them, improve the understanding and management of disease, and give back to communities through philanthropy and volunteerism.
Lilly employs approximately 41,000 people around the world, with more than 8,000 people engaged in research and development. Lilly’s research priorities are aligned with significant global health needs including diabetes, heart disease, mental health and cancer.
Lilly has been a global leader in diabetes care since 1923 and remains committed to helping people with diabetes through innovative science and support.
Lilly contributes to the identification of predictive biomarkers for glucose deterioration, the discovery of multi-omics biomarker and beta cell dysfunction biomarkers. Lilly co-leads WP3.
Lilly employs approximately 41,000 people around the world, with more than 8,000 people engaged in research and development. Lilly’s research priorities are aligned with significant global health needs including diabetes, heart disease, mental health and cancer.
Lilly has been a global leader in diabetes care since 1923 and remains committed to helping people with diabetes through innovative science and support.
Lilly contributes to the identification of predictive biomarkers for glucose deterioration, the discovery of multi-omics biomarker and beta cell dysfunction biomarkers. Lilly co-leads WP3.
RHAPSODY was a unique collaboration of leading public and private research groups, which has included over 100 researchers operating in 7 different scientific work packages. The RHAPSODY team was coordinated by the University of Lausanne, Servier and Lund University. RHAPSODY was based on the availability of large population prospective cohorts, with unique collection of genetic, biochemical and clinical data. RHAPSODY has also benefited from extensive and unique resources developed in previous IMI projects. Combining new and existing data with the expertise of its partners, RHAPSODY has developed novel biomarkers to refine diagnosis, leading to better patient stratification and with the aim of promoting diabetes prevention and supporting innovative drug discovery for personalized management of diabetes.
The RHAPSODY team is coordinated by the University of Lausanne, Servier and Lund University and is working on a new definition of the molecular taxonomy of T2D diabetes that will support patient segmentation, inform clinical trial design, and enable the establishment of regulatory paths for the adoption of novel strategies for diabetes prevention and treatment.




This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (www.imi.europa.eu) under grant agreement No 115881. This Joint Undertaking has received support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
This work has been supported by the Swiss State Secretariat for Education‚ Research and Innovation (SERI) under contract number 16.0097-2.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.
This work has been supported by the Swiss State Secretariat for Education‚ Research and Innovation (SERI) under contract number 16.0097-2.
The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.

Rhapsody is funded under the "Health" priority, which is an investment in our health and, on a larger scale, an investment in a healthy workforce, a healthy economy and lower public health bills.

IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe.
IMI is a joint undertaking between the European Union and the pharmaceutical industry association EFPIA.

The EFPIA Manager Research Partnerships plays a critical role in enabling participation of EFPIA members in collaborative research and development in the framework of private-private and public-private partnerships, such as but not limited to the Innovative Medicines Initiative or EU framework programmes.